Viral Antigens(病毒抗原)
A viral Antigen is an antigen with multiple antigenicities that is protein in nature, strain-specific, and closely associated with the virus particle. A viral antigen is a protein encoded by the viral genome.A viral protein is an antigen specified by the viral genome that can be detected by a specific immunological response.
Viruses are infectious pathogens that cause serious diseases & major threats for global public health, such as influenza, hepatitis, & AIDS. Virus is a sub-micrometer particle that has DNA or RNA packed in a shell called capsid. Viral antigens protrude from the capsid and often fulfill important function in docking to the host cell, fusion, and injection of viral DNA/RNA. Antibody-based immune responses form a first layer of protection of the host from viral infection; however, in many cases a vigorous cellular immune response mediated by T-cells and NK-cells is required for effective viral clearance. When cellular immunity is unable to clear the virus, the infection can become chronic, and serum antibodies to the viral pathogen are used as first indicator for the diagnosis of the disease.
ELISAs provide a valuable tool in the detection and diagnosis of virus infection. The ability to produce recombinant viral proteins will ensure that future ELISAs are safe, specific and rapid. Even when a virus cannot be cultured, provided gene sequence is available, it is possible to rapidly respond to emerging viruses and new viral strains of existing pathogens.
Recombinant viral antigens contain part of viral sequence meaning that the recombinant antigen contains a region which can be recognized by different antibodies produced by different individuals. This reduces the risk of false negatives which can occur with synthetic peptides, which contain only a small portion of the entire protein. If an individual infected with a viral antigen makes antibodies to a part of the protein not included in the synthetic peptides, a false negative results.
Recombinant viral protein usually contains a fusion protein/partner which produces superior attachment to assay surfaces such as wells. For this reason, smaller amounts of recombinant protein will produce the same results as larger amounts of unfused protein. The choice of fusion partner prevents false positives, allowing superior adhesion without incorrect results.
Recombinant Viral proteins are expressed in bacteria, yeast, mammalian cells, and viruses. E. Coli cells were first to be used for this purpose but the expressed proteins were not glycosylated, which was a major drawback since many of the immunogenic proteins of viruses such as the envelope glycoproteins, were glycosylated. Nevertheless, in many instances, it was demonstrated that the non-glycosylated protein backbone was just as immunogenic. The obvious advantage of recombinant viral antigens is that they are available in unlimited quantities and the production and quality control processes is simple.
Advantages of defined using recombinant viral antigens:
1. Production and quality control is simple.
2. No nucleic acids or other viral or external proteins, therefore less toxic.
3. Safer in cases where viruses are oncogenic or establish a persistent infection.
4. Feasible even if virus cannot be cultivated
Disadvantages:
1. May be less immunogenic than conventional inactivated whole-virus vaccines.
2. Requires adjuvant .
3. Fails to elicit CMI.
Facts about Viral Antigens:
1. A Viral Protein Mimics its Way into cells.
2. Viral Protein Helps Infected T Cells Stick To Uninfected Cells.
3. The Viral Protein A238L Inhibits Cyclooxygenase-2 Expression through a Nuclear Factor of Activated T Cell-dependent Trans-activation Pathway.
4. Viral Protein is an effective preventative against ear infection.
5. HIV-1 Viral Protein R Induces Apoptosis via a Direct Effect on the Mitochondrial Permeability Transition Pore.
6. The Level of Viral Antigen Presented by Hepatocytes Influences CD8 T-Cell Function.
7. Antigen-presenting cells from calves persistently infected with bovine viral diarrhea virus, a member of the Flaviviridae, are not compromised in their ability to present viral antigen.
8. There is a difference in the distribution and spread of a viral antigen, development of lesions and correlation between presence of viral antigen and lesions.
9. The absence of viral antigens on the surface of equine herpesvirus-1-infected peripheral blood mononuclear cells is a strategy to avoid complement-mediated lysis.
10. Viral Protein Influences Key Cell-signaling Pathway.
11. A viral protein produced by cancer-causing virus influences a key signaling pathway in the immune cells that the virus infects. This stimulates the cells to divide, helping the virus spread through the body.
12. Protection by recombinant viral proteins against a respiratory virulent avian metapneumovirus has been achieved.
13. Viral O-acetylesterases are found in influenza C viruses and Corona-viruses. Viral O-acetylesterases remove cellular receptors from the surface of target cells which destroys the receptor. Recombinant viral O-acetylesterases derived from Sf9 insect cells as chimeric proteins fused to eGFP specifically hydrolyze 9-O-acetylated sialic acids, while that of sialodacryoadenitis virus, a rat coronavirus related to mouse hepatitis virus, is specific for 4-O-acetylated sialic acid. The recombinant esterases were shown to specifically de-O-acetylate sialic acids on glycoconjugates. The recombinant viral proteins can be used to unambiguously identify O-acetylated acids.
Products for Viral Antigens
- Borrelia(28)
- Chagas(3)
- Chikungunya(7)
- Chlamydia(10)
- Cytomegalo(8)
- Dengue(50)
- Ebola(4)
- EBV(13)
- Encephalitis(8)
- Feline Leukemia Virus(1)
- Hantavirus(1)
- HBsAg(7)
- Helicobacter Pylori(3)
- Hepatitis A(15)
- Hepatitis B(10)
- Hepatitis C(85)
- Hepatitis D(1)
- Hepatitis E(5)
- Herpes(11)
- HERV-K(1)
- HIV(151)
- HTLV(6)
- Influenza(72)
- Lassa(2)
- Malaria(71)
- Mumps(1)
- Mycoplasma(4)
- Norovirus(4)
- Papillomavirus(5)
- Parvovirus(3)
- Rubella(3)
- S. Typhi(5)
- SARS(85)
- Shiga Like Toxin(2)
- Toxoplasma(9)
- Treponema(16)
- Varicella(3)
- West Nile(2)
- Zika(13)
- Cat.No. 产品名称 Information
-
GP25385
HIV-1 nef, Clade B
HIV-1 nef Clade B Recombinant

-
GP25392
HIV-1 p24
HIV-1 p24 重组体

-
GP25386
HIV-1 p24 Core
HIV-1 p24 Core Recombinant

-
GP25390
HIV-1 p24 gag
HIV-1 p24 gag Recombinant

-
GP25391
HIV-1 p24, 24 kDa
HIV-1 p24, 24 kDa Recombinant

-
GP25388
HIV-1 p24, Biotin
HIV-1 p24 Recombinant, Biotin Labeled

-
GP25387
HIV-1 p24, His
HIV-1 p24 Recombinant, His Tag

-
GP25389
HIV-1 p24, HRP
HIV-1 p24 Recombinant, HRP Labeled

-
GP25401
HIV-1 p55 gag
HIV-1 p55 gag Recombinant

-
GP25402
HIV-1 p66 pol
HIV-1 p66 pol Recombinant

-
GP25411
HIV-1 Protease
HIV-1 Protease Recombinant

-
GP25412
HIV-1 TAT Clade-A
HIV-1 TAT Clade-A Recombinant

-
GP25413
HIV-1 TAT Clade-B
HIV-1 TAT Clade-B Recombinant

-
GP25414
HIV-1 TAT Clade-C
HIV-1 TAT Clade-C Recombinant

-
GP25415
HIV-1 TAT Clade-D
HIV-1 TAT Clade-D Recombinant

-
GP25417
HIV-1 TAT Cys22
HIV-1 TAT Cys22 Recombinant

-
GP25416
HIV-1 TAT, Biotin
HIV-1 TAT Recombinant, Biotin Labeled

-
GP25418
HIV-1, 2
HIV-1 envelope conjugated to HIV-2 gp39 Recombinant

-
GP25428
HIV-2 Envelope
HIV-2 Envelope Recombinant

-
GP25427
HIV-2 gp-36 397aa
HIV-2 gp36 397aa Recombinant

-
GP26442
HIV-2 gp160
HIV-2 gp160 produced in E

-
GP25422
HIV-2 gp32
HIV-2 gp32 Recombinant

-
GP25423
HIV-2 gp32, Biotin
HIV-2 gp32 Recombinant, Biotin Labeled

-
GP25424
HIV-2 gp32, HRP
HIV-2 gp32 Recombinant, HRP Labeled

-
GP25425
HIV-2 gp36
Synthetic HIV-2 gp36

-
GP25426
HIV-2 gp36, 17kDa
HIV-2 gp36 Recombinant

-
GP25429
HIV-2 Protease
HIV-2 Protease Recombinant

-
GC65134
HIV-IN-6
HIV-IN-6 是一种 HIV-Ⅰ 病毒复制抑制剂,通过靶向与病毒 Nef 蛋白相互作用的 Src 家族激酶 (SFK) (如 Hck) 而起作用。
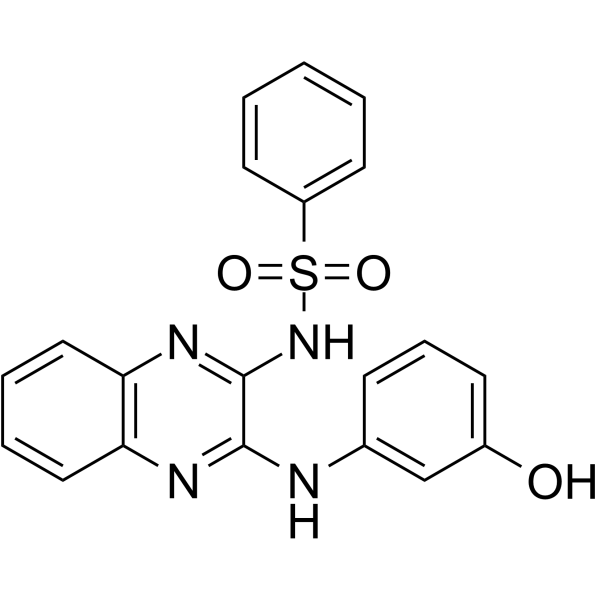
-
GP23628
HP-NAP
Neutrophil-activating protein A Helicobacter Pylori Recombinant

-
GP25440
HPV 11
Human Papillomavirus 11 Recombinant

-
GP25437
HPV 16
Human Papillomavirus 16 Recombinant

-
GP25438
HPV 18
Human Papillomavirus 18 Recombinant

-
GP25439
HPV 6
Human Papillomavirus 6 Recombinant

-
GP25441
HPV16 E6
Human Papillomavirus 16 E6 Recombinant

-
GP25207
HSV-1 gD
Herpes Simplex Virus-1 gD Recombinant

-
GP25208
HSV-1 gD (84-175)
Herpes Simplex Virus-1 gD (84-175 a.a.) Recombinant

-
GP25209
HSV-1 gG
Herpes Simplex Virus-1 gG Recombinant

-
GP25216
HSV-2 gB
Herpes Simplex Virus-2 gB Recombinant

-
GP25210
HSV-2 gD
Herpes Simplex Virus-2 gD Recombinant

-
GP25212
HSV-2 gD (31-335)
Herpes Simplex Virus-2 gD (31-335 a.a.) Recombinant

-
GP25211
HSV-2 gD (525-578)
Herpes Simplex Virus-2 gD (525-578 a.a.) Recombinant

-
GP25213
HSV-2 gG
Herpes Simplex Virus-2 gG Recombinant

-
GP25217
HSV-2 VP13/14
Herpes Simplex Virus-2 VP13/14 Recombinant

-
GP25218
HSV-2 VP22
Herpes Simplex Virus-2 VP22 Recombinant

-
GP25215
HSV-8 M
Herpes Simplex Virus-8 Mosaic Recombinant

-
GP25431
HTLV-1 Envelope
HTLV-1 Envelope Recombinant

-
GP25434
HTLV-1 gp21
HTLV-1 gp21 Recombinant

-
GP25435
HTLV-1 gp46 mosaic
HTLV-I gp46 Mosaic Recombinant

-
GP25436
HTLV-1 mosaic
HTLV-1 Mosaic Recombinant

-
GP25433
HTLV-1 p24
HTLV-1 p24 Recombinant





