Viral Antigens(病毒抗原)
A viral Antigen is an antigen with multiple antigenicities that is protein in nature, strain-specific, and closely associated with the virus particle. A viral antigen is a protein encoded by the viral genome.A viral protein is an antigen specified by the viral genome that can be detected by a specific immunological response.
Viruses are infectious pathogens that cause serious diseases & major threats for global public health, such as influenza, hepatitis, & AIDS. Virus is a sub-micrometer particle that has DNA or RNA packed in a shell called capsid. Viral antigens protrude from the capsid and often fulfill important function in docking to the host cell, fusion, and injection of viral DNA/RNA. Antibody-based immune responses form a first layer of protection of the host from viral infection; however, in many cases a vigorous cellular immune response mediated by T-cells and NK-cells is required for effective viral clearance. When cellular immunity is unable to clear the virus, the infection can become chronic, and serum antibodies to the viral pathogen are used as first indicator for the diagnosis of the disease.
ELISAs provide a valuable tool in the detection and diagnosis of virus infection. The ability to produce recombinant viral proteins will ensure that future ELISAs are safe, specific and rapid. Even when a virus cannot be cultured, provided gene sequence is available, it is possible to rapidly respond to emerging viruses and new viral strains of existing pathogens.
Recombinant viral antigens contain part of viral sequence meaning that the recombinant antigen contains a region which can be recognized by different antibodies produced by different individuals. This reduces the risk of false negatives which can occur with synthetic peptides, which contain only a small portion of the entire protein. If an individual infected with a viral antigen makes antibodies to a part of the protein not included in the synthetic peptides, a false negative results.
Recombinant viral protein usually contains a fusion protein/partner which produces superior attachment to assay surfaces such as wells. For this reason, smaller amounts of recombinant protein will produce the same results as larger amounts of unfused protein. The choice of fusion partner prevents false positives, allowing superior adhesion without incorrect results.
Recombinant Viral proteins are expressed in bacteria, yeast, mammalian cells, and viruses. E. Coli cells were first to be used for this purpose but the expressed proteins were not glycosylated, which was a major drawback since many of the immunogenic proteins of viruses such as the envelope glycoproteins, were glycosylated. Nevertheless, in many instances, it was demonstrated that the non-glycosylated protein backbone was just as immunogenic. The obvious advantage of recombinant viral antigens is that they are available in unlimited quantities and the production and quality control processes is simple.
Advantages of defined using recombinant viral antigens:
1. Production and quality control is simple.
2. No nucleic acids or other viral or external proteins, therefore less toxic.
3. Safer in cases where viruses are oncogenic or establish a persistent infection.
4. Feasible even if virus cannot be cultivated
Disadvantages:
1. May be less immunogenic than conventional inactivated whole-virus vaccines.
2. Requires adjuvant .
3. Fails to elicit CMI.
Facts about Viral Antigens:
1. A Viral Protein Mimics its Way into cells.
2. Viral Protein Helps Infected T Cells Stick To Uninfected Cells.
3. The Viral Protein A238L Inhibits Cyclooxygenase-2 Expression through a Nuclear Factor of Activated T Cell-dependent Trans-activation Pathway.
4. Viral Protein is an effective preventative against ear infection.
5. HIV-1 Viral Protein R Induces Apoptosis via a Direct Effect on the Mitochondrial Permeability Transition Pore.
6. The Level of Viral Antigen Presented by Hepatocytes Influences CD8 T-Cell Function.
7. Antigen-presenting cells from calves persistently infected with bovine viral diarrhea virus, a member of the Flaviviridae, are not compromised in their ability to present viral antigen.
8. There is a difference in the distribution and spread of a viral antigen, development of lesions and correlation between presence of viral antigen and lesions.
9. The absence of viral antigens on the surface of equine herpesvirus-1-infected peripheral blood mononuclear cells is a strategy to avoid complement-mediated lysis.
10. Viral Protein Influences Key Cell-signaling Pathway.
11. A viral protein produced by cancer-causing virus influences a key signaling pathway in the immune cells that the virus infects. This stimulates the cells to divide, helping the virus spread through the body.
12. Protection by recombinant viral proteins against a respiratory virulent avian metapneumovirus has been achieved.
13. Viral O-acetylesterases are found in influenza C viruses and Corona-viruses. Viral O-acetylesterases remove cellular receptors from the surface of target cells which destroys the receptor. Recombinant viral O-acetylesterases derived from Sf9 insect cells as chimeric proteins fused to eGFP specifically hydrolyze 9-O-acetylated sialic acids, while that of sialodacryoadenitis virus, a rat coronavirus related to mouse hepatitis virus, is specific for 4-O-acetylated sialic acid. The recombinant esterases were shown to specifically de-O-acetylate sialic acids on glycoconjugates. The recombinant viral proteins can be used to unambiguously identify O-acetylated acids.
Products for Viral Antigens
- Borrelia(28)
- Chagas(3)
- Chikungunya(7)
- Chlamydia(10)
- Cytomegalo(8)
- Dengue(50)
- Ebola(4)
- EBV(13)
- Encephalitis(8)
- Feline Leukemia Virus(1)
- Hantavirus(1)
- HBsAg(7)
- Helicobacter Pylori(3)
- Hepatitis A(15)
- Hepatitis B(10)
- Hepatitis C(85)
- Hepatitis D(1)
- Hepatitis E(5)
- Herpes(11)
- HERV-K(1)
- HIV(151)
- HTLV(6)
- Influenza(72)
- Lassa(2)
- Malaria(71)
- Mumps(1)
- Mycoplasma(4)
- Norovirus(4)
- Papillomavirus(5)
- Parvovirus(3)
- Rubella(3)
- S. Typhi(5)
- SARS(85)
- Shiga Like Toxin(2)
- Toxoplasma(9)
- Treponema(16)
- Varicella(3)
- West Nile(2)
- Zika(13)
- Cat.No. 产品名称 Information
-
GP25296
HCV NS5 Genotype-3
Hepatitis C Virus NS5 Genotype-3 Recombinant

-
GP25297
HCV NS5 Genotype-3a
Hepatitis C Virus NS5 Genotype-3a Recombinant

-
GP25298
HCV NS5 Genotype-3b
Hepatitis C Virus NS5 Genotype-3b Recombinant

-
GP25299
HCV NS5 Genotype-4
Hepatitis C Virus NS5 Genotype-4 Recombinant

-
GP25300
HCV NS5 Genotype-5
Hepatitis C Virus NS5 Genotype-5 Recombinant

-
GP25301
HCV NS5 Genotype-6
Hepatitis C Virus NS5 Genotype-6 Recombinant

-
GP25302
HCV NS5 Genotype-6a
Hepatitis C Virus NS5 Genotype-6a Recombinant

-
GP25307
HCV NS5, Biotin
Hepatitis C Virus NS5, Biotin Recombinant

-
GP25308
HCV NS5, HRP
Hepatitis C Virus NS5, Horseradish Peroxidase Recombinant

-
GP25304
HCV NS5A
Hepatitis C Virus NS5A Recombinant

-
GP25305
HCV NS5B
Hepatitis C Virus NS5B Recombinant

-
GP25306
HCV NS5B (2634-2752 a.a)
Hepatitis C Virus NS5B (2634-2752 a.a) Recombinant

-
GP25338
HDV
Hepatitis D Virus Recombinant

-
GC43818
Herquline A
梅花青霉素A,Herqueline A
An alkaloid fungal metabolite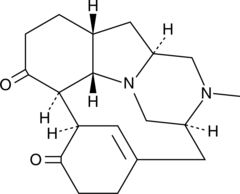
-
GP25522
HERV-K
Endogenous retrovirus K Envelope Human Recombinant

-
GP25339
HEV Mosaic
Hepatitis E Virus Mosaic Recombinant

-
GP25341
HEV ORF2 (403-461 a.a.)
Hepatitis E Virus ORF2 (403-461 a.a.) Recombinant

-
GP25342
HEV ORF2 (452-617 a.a.)
Hepatitis E Virus ORF2 (452-617 a.a.) Recombinant

-
GP25340
HEV ORF2 (633-659 a.a.)
Hepatitis E Virus ORF2 (633-659 a.a.) Recombinant

-
GP25343
HEV ORF3
Hepatitis E Virus ORF3 Recombinant

-
GP25419
HIV Type-O Envelope
HIV Type-O Envelope

-
GP25420
HIV Type-O gp41
HIV Type-O gp41 Recombinant

-
GP25421
HIV Type-O gp41 13kDa
HIV Type-O gp41 13kDa Recombinant

-
GP26440
HIV-1 CRF
HIV-1 CRF Recombinant produced in E

-
GP25383
HIV-1 Envelope
HIV-1 Envelope Recombinant

-
GP26441
HIV-1 GAG
HIV-1 GAG Recombinant produced in E

-
GP25406
HIV-1 gag p17, p24
HIV-1 gag p17, p24 Recombinant

-
GP25407
HIV-1 gag p17,p24, gp120
HIV-1 gag p17,p24, gp120 Recombinant

-
GP25408
HIV-1 gag p17-p24, gp41-gp120
HIV-1 gag p17-p24, gp41-gp120 Recombinant

-
GP25404
HIV-1 gp120 LAV
HIV-1 gp120 LAV Envelope Recombinant

-
GP25403
HIV-1 gp120 Nef Mosaic
HIV-1 gp120 Nef Mosaic Recombinant

-
GP25393
HIV-1 gp41
HIV-1 gp41 Recombinant

-
GP25399
HIV-1 gp41 16kDa
HIV-1 gp41 16kDa Recombinant

-
GP25396
HIV-1 gp41 Long
HIV-1 gp41 Long Recombinant

-
GP25397
HIV-1 gp41 Long, Biotin
HIV-1 gp41 Long Recombinant, Biotin Labeled

-
GP25398
HIV-1 gp41 Long, HRP
HIV-1 gp41 Long Recombinant, HRP Labeled

-
GP26438
HIV-1 gp41 Subtype-b
Recombinant HIV-1 gp41 Subtype-b produced in E

-
GP26439
HIV-1 gp41 Subtype-c
Recombinant HIV-1 gp41 Subtype-c produced in E

-
GP25394
HIV-1 gp41, Biotin
HIV-1 gp41 Recombinant, Biotin labeled

-
GP25395
HIV-1 gp41, HRP
HIV-1 gp41 Recombinant, HRP labeled

-
GP25400
HIV-1 gp41/120
HIV-1 gp41-gp120 Mosaic Recombinant

-
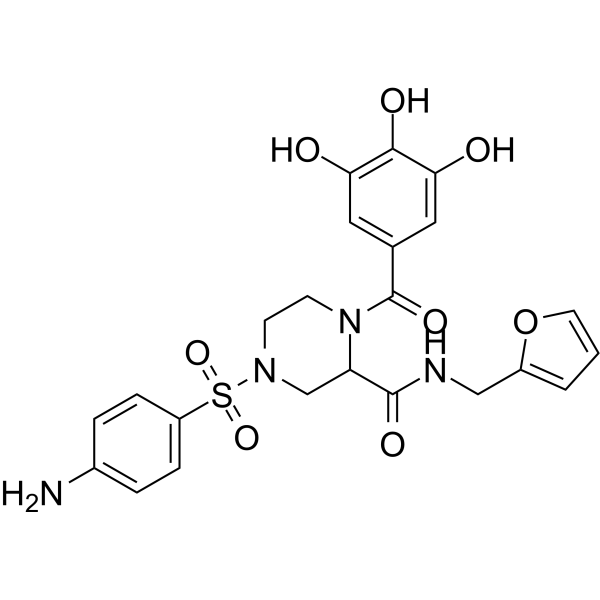
GC69230
HIV-1 inhibitor-45
HIV-1 inhibitor-45 (compound IA-6) 是一种有效的 HIV-1 RNase H 抑制剂,IC50 为 0.067 μM。HIV-1 inhibitor-45 显示出抗病毒活性。
-
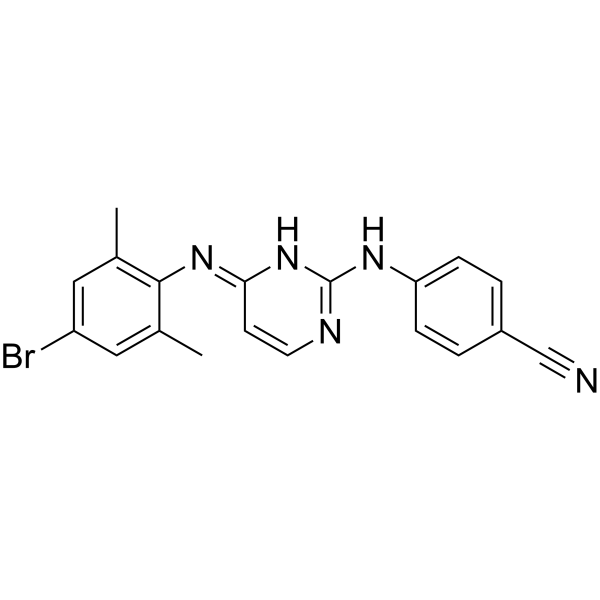
GC68023
HIV-1 inhibitor-48
HIV-1 inhibitor-48 (compound 13o) 是一种新型的非核苷逆转录酶抑制剂 (NNRTI),具有抗 HIV-1 活性。
-
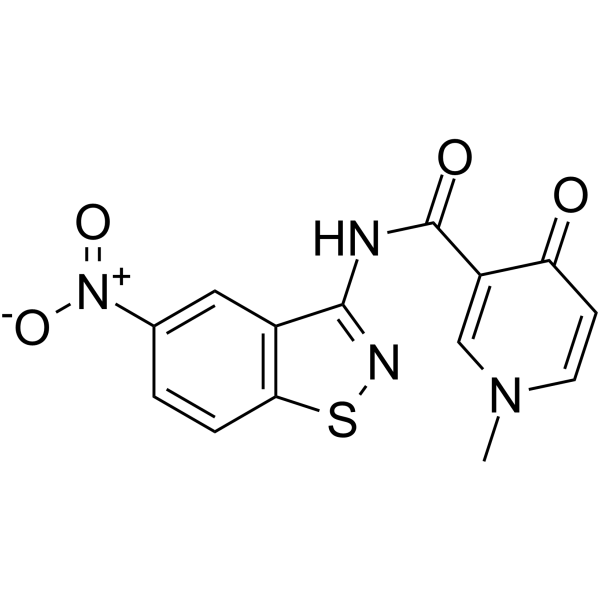
GC65256
HIV-1 inhibitor-6
HIV-1 inhibitor-6 (compound 9) 是一种基于二杂芳基酰胺的化合物,是一种有效的 HIV-1 pre-mRNA 选择性剪接抑制剂。HIV-1 inhibitor-6 可以阻断 HIV 复制。HIV-1 inhibitor-6 对野生型 HIV-1IIIB (亚型 B,X4-tropic) 和 HIV-1 97USSN54 (亚型 A,R5-tropic) 具有活性,EC50 值分别为 0.6 μM 和 0.9 μM。HIV-1 inhibitor-6 抑制对靶向 HIV 逆转录酶、蛋白酶、整合酶和辅助受体 CCR5 的药物耐药的 HIV 菌株,EC50 范围为 0.9 至 1.5 μM。
-
GC74067
HIV-1 inhibitor-69
HIV-1 inhibitor-69 (compound Test set 1)是一种HIV-1 RT抑制剂。
-
GC65158
HIV-1 inhibitor-8
HIV-1 inhibitor-8 是一种具有口服活性、低毒和有效的 HIV -1 非核苷逆转录酶抑制剂 (NNRTI)。HIV-1 inhibitor-8 对各种 HIV -1 菌株产生有效的抗病毒活性 (EC50=4.44~54.5 nM)。HIV-1 inhibitor-8 对 WT HIV-1 逆转录酶的 IC50 为 0.081 μM。
-
GP25409
HIV-1 Integrase
HIV-1 Integrase Recombinant

-
GP25410
HIV-1 Integrase p31
HIV-1 Integrase p31 Recombinant

-
GP25384
HIV-1 nef
HIV-1 nef Recombinant

-
GP26437
HIV-1 NEF Biotin
Recombinant HIV-1 nef Biotin Labeled is a full length protein produced in E





